With the rapid development of consumer electronics, electric vehicles, and energy storage, existing lithium-ion batteries are unable to meet the growing demand for energy density. In the current lithium-ion battery system, the mismatch between anode and cathode materials seriously hinders the development of lithium-ion batteries. Therefore, cathode electrode materials are the key to improving the energy density of lithium-ion batteries. At present, the commercialized cathode materials include LiCoO2, LiMn2O4, LiFePO4 and LiMO2 (M=Ni, Co, Mn/Al). However, its actual capacity has approached its theoretical value and is difficult to meet the growing market demand, so it is urgent to explore a new generation of cathode materials with low-cost, high-energy density and high safety.
Li-rich Mn-based cathode materials have outstanding advantages such as high specific capacity (>250 mAh/g), high operating voltage (>4.5 V), low production cost and environmental protection. They are key electrode materials for lithium-ion batteries with energy density exceeding 400Wh/kg, and are considered to be the most promising candidate materials for the next generation of lithium-ion battery cathode materials. The existence of reversible cation and anion redox reactions in Li-rich Mn-based cathode materials is the key to obtain high specific capacity. However, at the cut-off voltages of 4.6-4.8 V vs Li/Li+, the activation of Li2MnO3 phase during first cycle charge leads to irreversible Li+ removal and oxygen release, resulting in its low initial coulombic efficiency. Irreversible oxygen release and structural phase transition during cycling leads to severe capacity and voltage decay. Poor electronic and ionic conductivity leads to reduced rate performance. These problems severely limit their further applications.
To address the paradoxical problem of high density energy storage in Li-rich Mn-based cathode materials versus maintaining structural integrity and long service life during charge/discharge cycles, our laboratory has introduced additional strong Al-O bonds in the local atomic coordination around O and TM by doping the Co-free Li-rich materials with Al elements, which can effectively modulate the local electronic structure and shift the TM 3d-O 2p and O 2p non-bonding bands to lower energies, increasing the potential for redox reactions (Fig. 1a,b). In addition, the introduction of Al enhances the interaction with oxygen, increases the oxygen vacancy formation energy and effectively enhances the reversibility of oxygen redox. The prepared electrode material can achieve a discharge specific capacity of 251.4 mAh g-1 at 0.1 C. The capacity retention rate can still maintain 78.8% after 300 cycles at 1 C (Fig. 1c). Our work provides an efficient and scalable strategy for the preparation of electrode materials with high voltages and high specific energy densities, making them highly promising candidates as cathode materials for lithium-ion batteries. These results were published in Journal of Alloys and Compounds, 2023.1694.
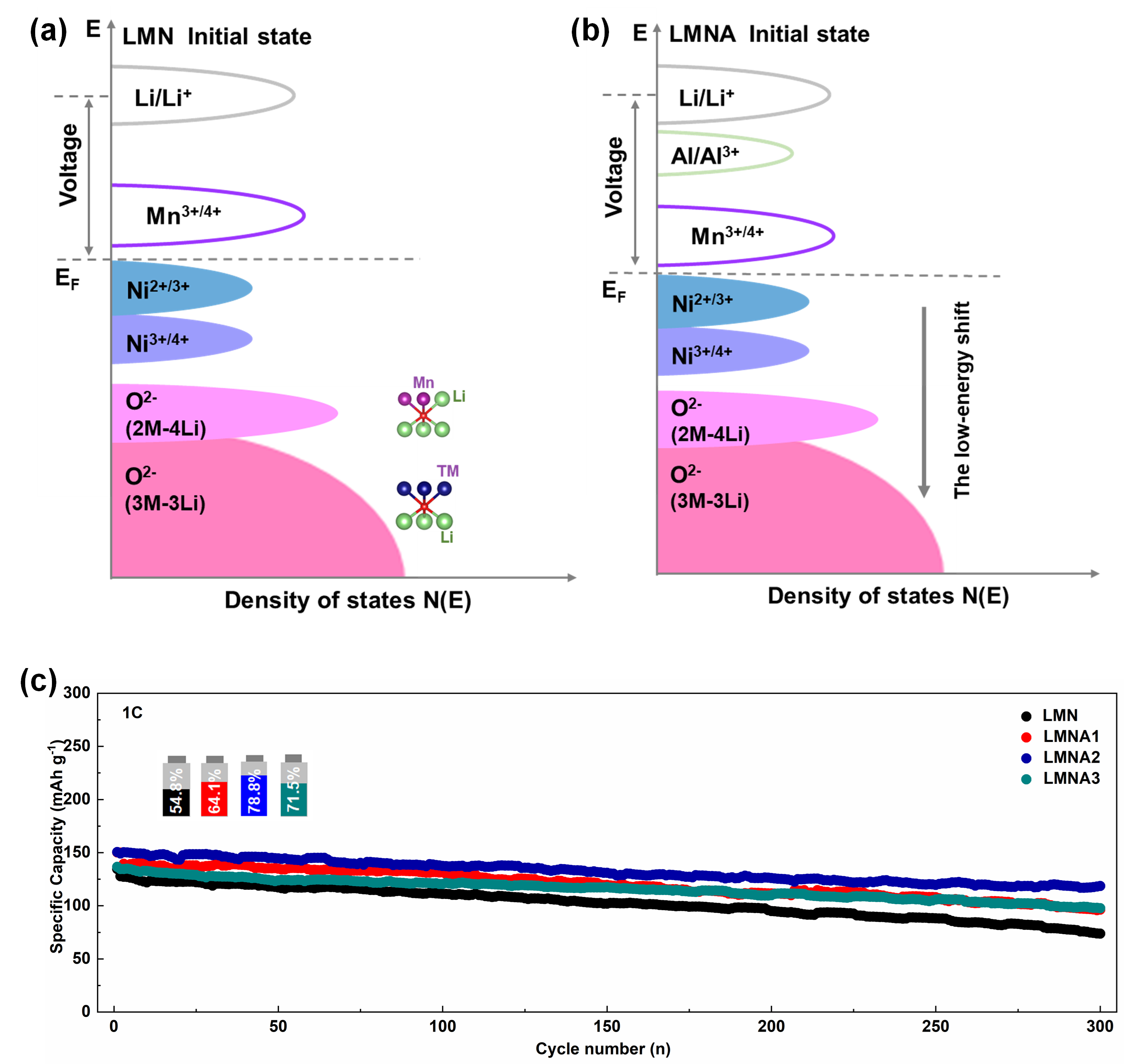
Fig.1. Schematic diagram of the energy band structure of Co-free Li-rich Mn-based cathode: (a) Li1.2Ni0.2Mn0.6O2 (LMN), (b) Li1.2Ni0.2Mn0.6-xAlxO2 (LMNA). (c) Cycling performance at 1 C current density
In response to the bottleneck issue of irreversible oxygen release during the cycling process of Li-rich Mn-based cathode materials, our team proposes a facile synchronous lithiation strategy combining the advantages of yttrium doping and LiYO2 surface coating. As shown in Fig. 2a, the Li+ conductor LiYO2 nanocoating reduces surface side reactions, inhibits the dissolution of transition metals, and improves the conductivity of lithium ions. The Y-O strong bond energy can bind the TM-O layer, stabilize and strengthen the lattice in the deep delithization state (>4.3 V), improve electrochemical reversibility and cycling stability, and reduce voltage decay during lithiation/delithization cycles. As illustrated in Fig. 2b, the designed cathode material LiYO2@LRM exhibits a uniform secondary micron spherical morphology formed by the accumulation of primary nanoparticles, resulting in a very high compaction density (≥3.0 g cm-3) of the prepared Li-rich Mn-based cathode material, which gives it a high specific energy density. The specific capacity is close to 250 mAh g-1 at 0.1C, and the capacity retention rate can reach more than 78.1% after 300 cycles at 1 C. The results were published in Green Energy & Environment, 2022.
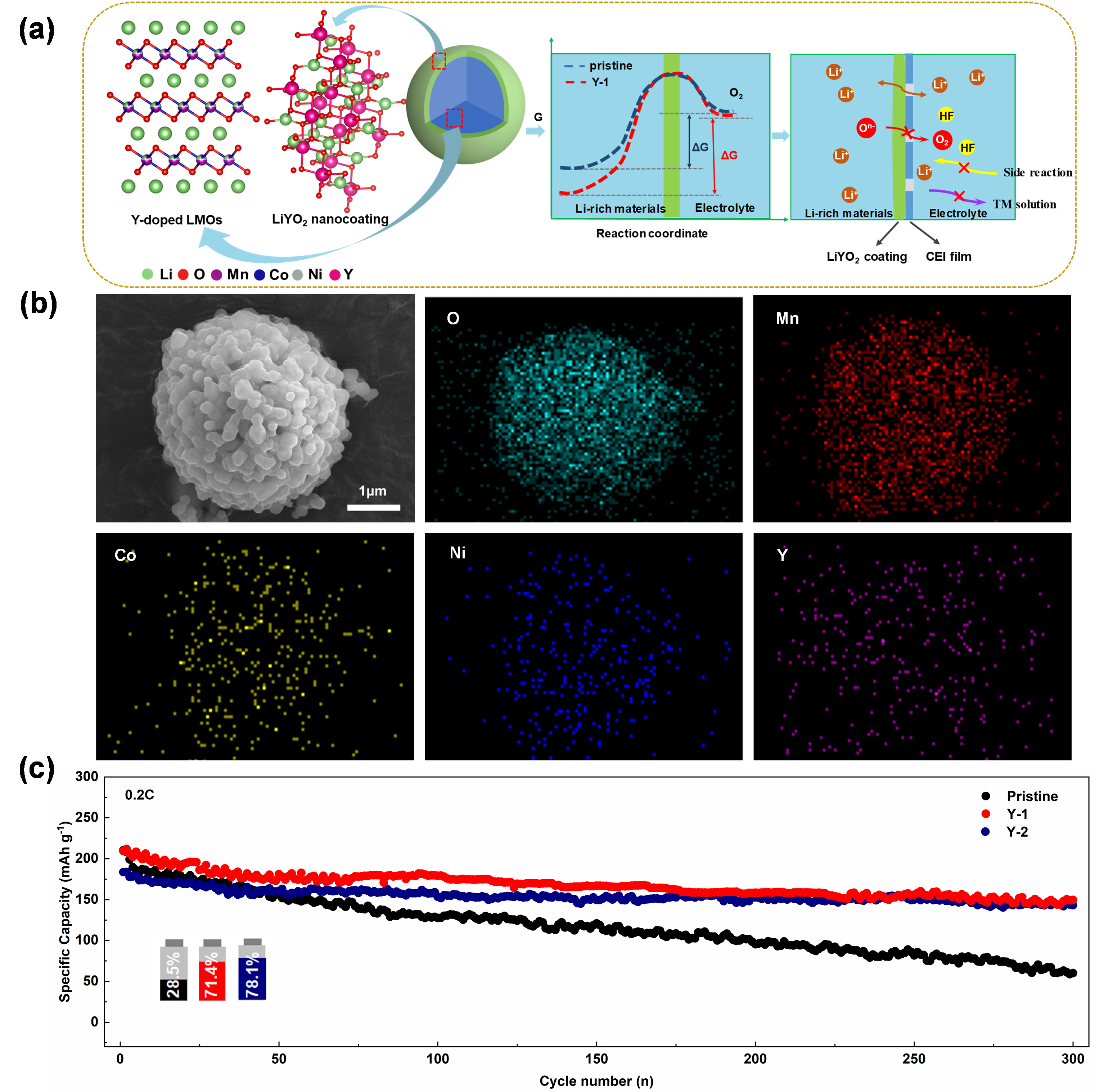
Fig. 2. (a) Schematic diagram of the effect of the simultaneous lithiation strategy; (b) morphology of the modified samples; (c) Cycling performance of the samples at 1 C.
Industrialization:
After years of deep cultivation, the modified cathode material designed by our group can reach a specific capacity of 250 mAh g-1 at a current density of 0.1 C, with a capacity retention rate of over 80% after 500 cycles. We have assembled this cathode material with a commercial graphite anode into a full battery and measured a specific capacity of 236.5 mAh g-1 at a current density of 0.1C, with an energy density of 400 Wh kg-1. At the same time, the Li-rich Mn-based cathode material assembled into a full battery in this study has a higher specific capacity and energy density of 230-250 mAh g-1 and 280 Wh kg-1, respectively, with excellent cycling performance compared to current mainstream commercial cathode materials, and a lower cost of approximately 150,000/ton compared to commercial ternary cathode materials (Fig. 3).
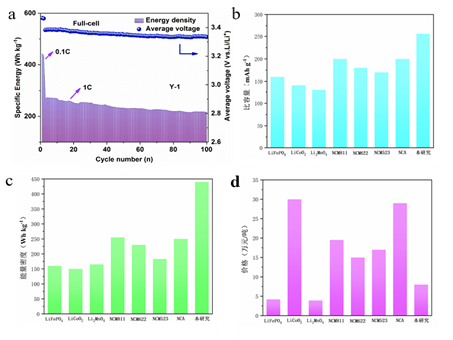
Fig. 3. Comparison of the performance of commercial cathode materials with the cathode materials in this study

