Based on the kinetic slow oxygen reduction reaction, the team is committed to developing low-cost, high-activity platinum-free catalysts in response to the key issues of high cost and poor cycling stability of platinum-based catalysts. With material structure design and active site construction as the main focus, we use hot filament vapour deposition, electrostatic spinning, high temperature pyrolysis and hot arc plasma to regulate the preparation of different systems of platinum-free catalysts, analyse the intrinsic link between microstructure, intrinsic activity and catalytic performance, explore in depth key scientific issues such as catalytic mechanism, providing new strategies for high-performance platinum-free catalysts.
The monoatomic catalysts are structurally homogeneous, maximise metal atom utilisation efficiency and have uniquely high activity. The Nb single-atom mosaic graphite nanostructures prepared by the DC arc physics method possess superior catalytic ability over noble metals for oxygen reduction reactions due to their d-orbital electronic reconfiguration. The nanostructures exhibit outstanding catalytic stability during long service life. Heteroatom-doped carbon materials are of interest due to their high catalytic activity, good stability and methanol tolerance. Different carbon-based materials prepared using hot filament chemical vapour deposition, where the heteroatom doping changes the electronic structure of the carbon material, exhibit better catalytic stability. For high performance nitrogen-doped carbon systems, pyridine nitrogen and graphite nitrogen were considered as effective active sites; the first proposal to use electrostatic spinning with molten salt assisted phase to design and develop nitrogen-rich one-dimensional cross-linked mesoporous carbon-based catalysts with ultra-high surface area (1069 m2/g) greatly enhanced the mass transfer performance and the number of active sites, showing excellent catalytic activity (E1/2=0.79 V). N-doped carbon-loaded transition metal (M-N-C) catalysts, especially Fe-N-C, are among the most promising Pt/C alternatives due to their high activity and stability. The interposed monatomic Fe-NX active sites and nanoparticles synergistically promote the ORR reaction rate. Our team prepared a highly loaded metal catalyst for N-CNTs (Fe/Ce-NCNTs) by introducing rare earth cerium into the Fe-based catalyst. The synthesized Fe/Ce-NCNTs catalysts are rich in multiple active sites and demonstrate excellent ORR performance in alkaline media (E1/2 = 0.86 V) with good electrochemical performance in aluminium-air battery applications.
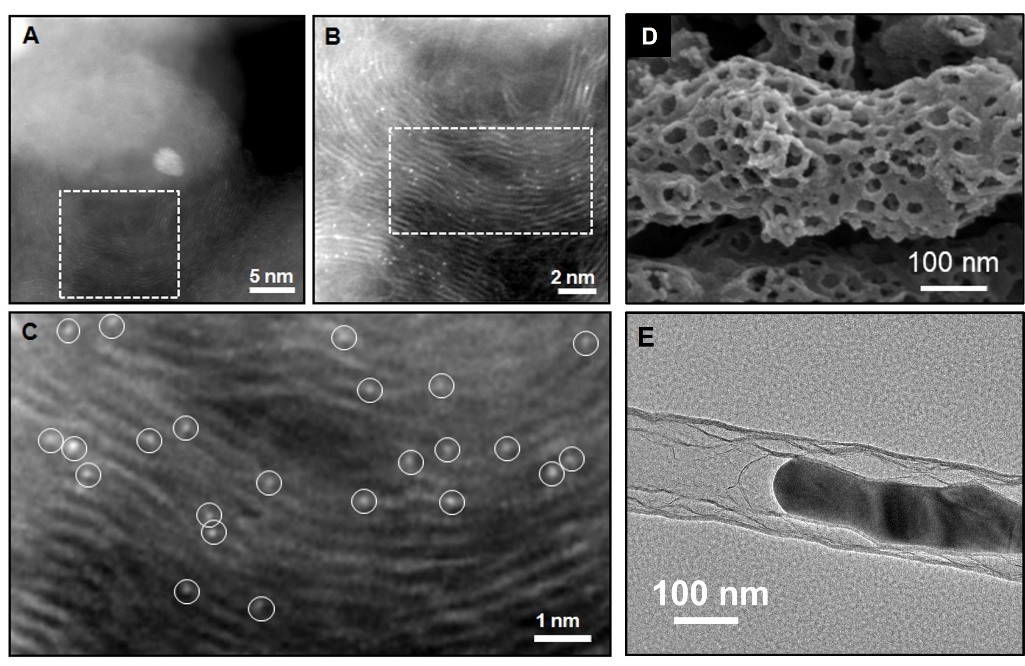
Figure 1. (A-C) Nb single-atom mosaic graphite nanostructures, (D) Nitrogen-rich doped one-dimensional cross-linked mesoporous carbon-based catalysts, (E) N-CNTs highly loaded metal catalysts

