This research direction is based on the design method of material composition at the atomic scale, theoretical calculation of phase composition and chemical composition in key materials for energy storage, and provides the theoretical basis for the determination of preparation process parameters at a later stage; using first principles, molecular dynamics and other computational methods, the multi-level precision combination calculation of key materials for energy storage is oriented to functional requirements, and theoretically validate the universality of the design method in the energy field and provide theoretical guidance for energy storage material performance prediction.
The effect of two-dimensional Ti2C MXene and its cut-off surface on catalytic activity was investigated in the laboratory. Based on the role of the surface oxide layer on the catalytic activity of TiC, the catalytic activity of Ti2C MXene and Ti2C MXene cut-off by O-, F- and OH-, respectively, was investigated for the two-dimensional Ti2C MXene, which necessarily has an O-cut-off surface and a larger specific surface area during the experimental preparation, and the results showed that the O atom exhibited the strongest adsorption ability, followed by the F, OH and H groups. The trend of catalytic activity is consistent with the trend of adsorption energy, i.e., Ti2CO2>Ti2CF2>Ti2C(OH)2>Ti2C. O- cut-off Ti2C (Ti2CO2) has the best catalytic activity with the lowest ORR and OER overpotential of 0.10 and 0.16 V, respectively, and its Ti-3d orbitals near the Fermi energy level are completely polarized, leading to its oxidation O22-strong oxidation properties. The presence of unfavorable functional groups should be minimized in the experiment, and more synthesis of two-dimensional Ti2C surfaces with O-cutoffs provides an important guiding role for the experimental preparation.
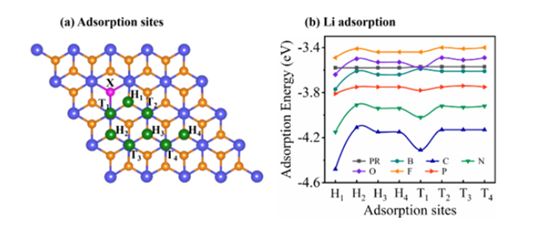
Figure 1. adsorption configurations and adsorption energies of different systems
The laboratory addresses the bottleneck problem that the performance of two-dimensional transition metal sulfide (TMDs) is limited by spontaneous aggregation and unstable electrochemical processes in the energy storage process of the conversion reaction, and investigates the effects of doping and defects on the adsorption diffusion and conductivity of alkali metals on TMDs, designs electrode materials from the atomic level and predicts the performance of battery applications, and by comparing the adsorption energy of alkali metals in the doped TiS2 system can The high capacity materials can be selected by comparing the alkali metal adsorption energy of TiS2 doped system (Figure 1). Meanwhile, the charge density difference maps of B-doped and defective TiS2 systems can visualize the electron transfer process and reveal the interactions. The above work provides a basic understanding of the effect of doping and defects on the electronic properties and alkali metal storage properties of TMDs, which provides an important guiding role for the later functional requirements, and the related results have been published in Journal of Physical Chemistry C, Applied Surface Science and other journals.

